_0.jpg?itok=RuBFwyf1)
Le modèle atomique de RutherfordBohr Alloprof
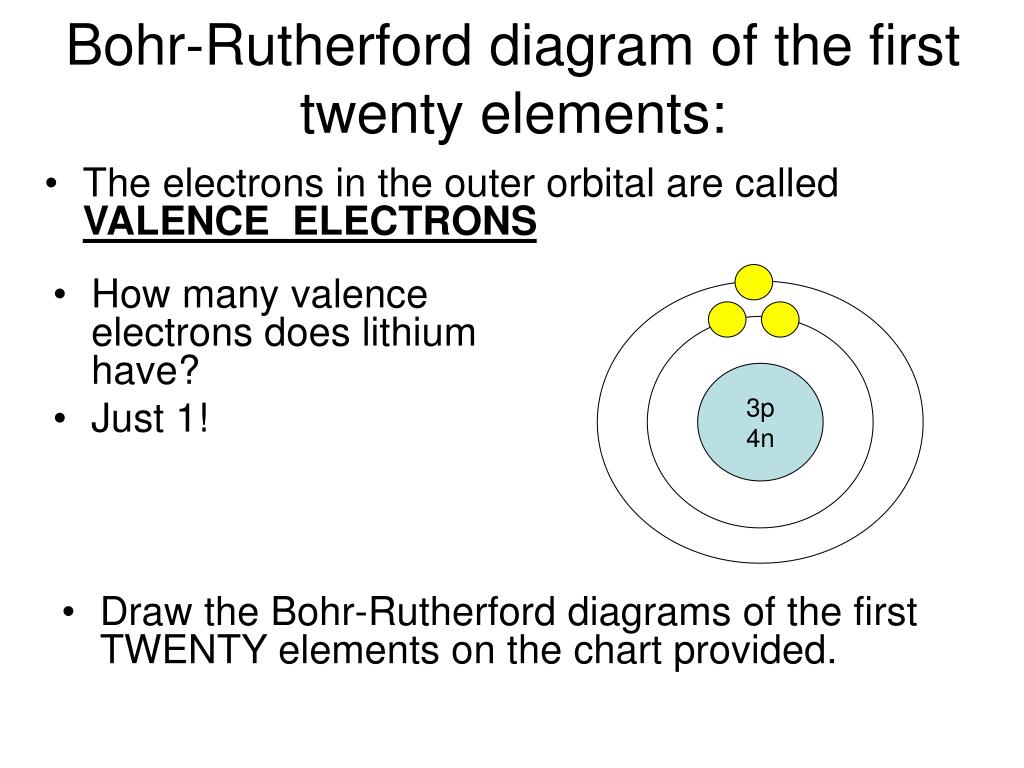
Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:

PPT Bohr Rutherford Atomic Model PowerPoint Presentation, free
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.
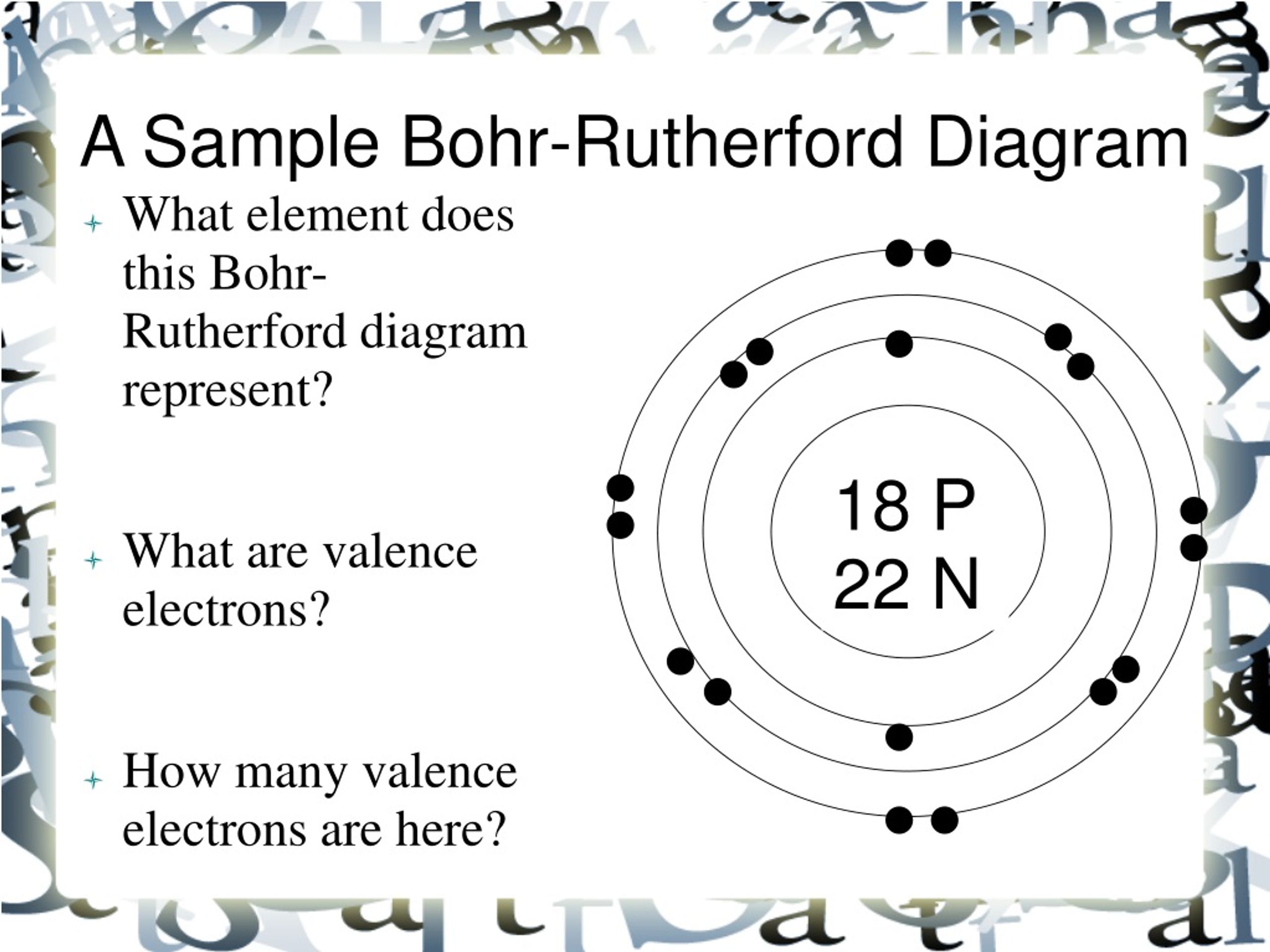
PPT BohrRutherford Diagrams PowerPoint Presentation, free download
More. Embed this widget ». Added Aug 1, 2010 by JB1295 in Chemistry. Gives the Lewis Dot structure for any element. Send feedback | Visit Wolfram|Alpha. Lewis Dot Diagram of. Submit. Get the free "Bohr Model Widget" widget for your website, blog, Wordpress, Blogger, or iGoogle.

Bohr's Atomic Model — Overview & Importance Expii
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.
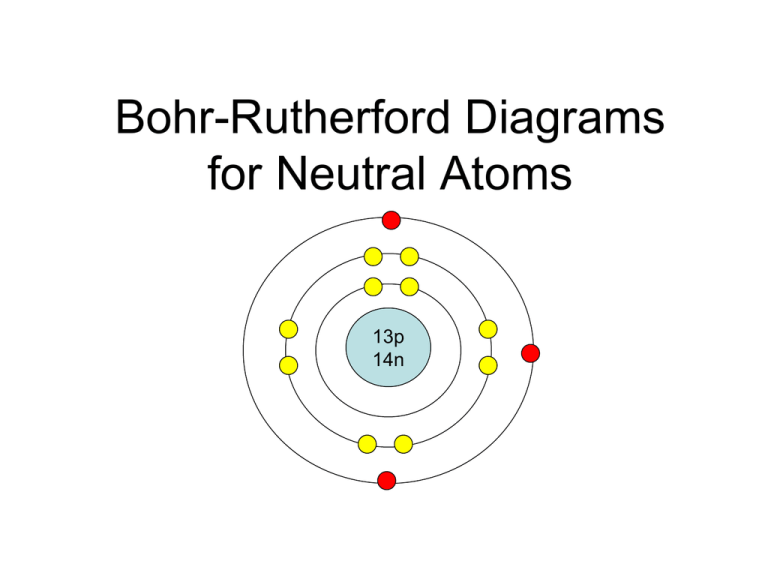
BohrRutherford diagrams for atoms
Rutherford atomic model, nuclear atom, or planetary model of the atom Key People: Ernest Rutherford atom Top Questions What was the impact of Ernest Rutherford's theory? Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.
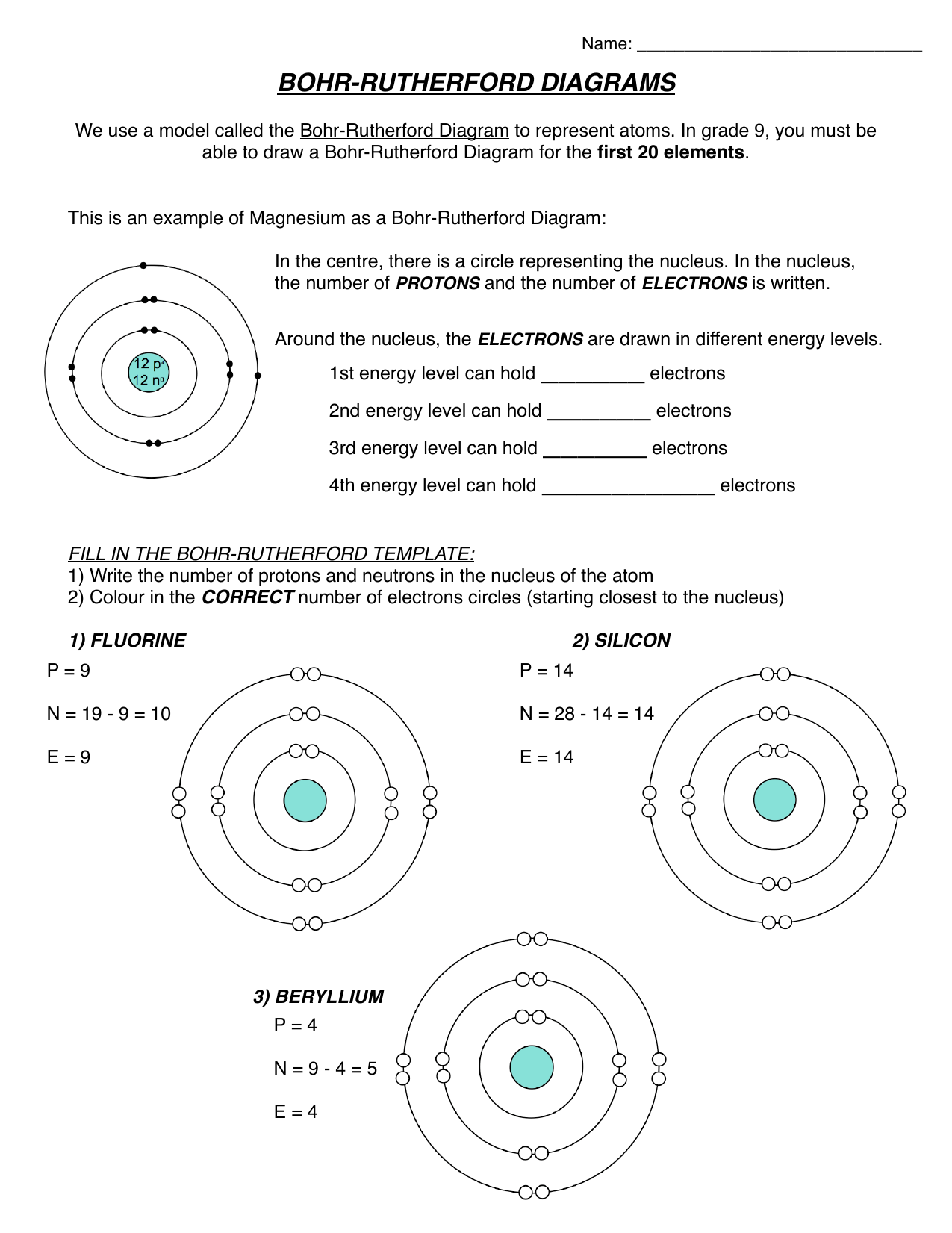
Bohr Rutherford Diagram For First 20 Elements General Wiring Diagram
How to Draw the Bohr-Rutherford Diagram for Calcium chemistNATE 260K subscribers Subscribe Subscribed 990 92K views 4 years ago Calcium has 2 electrons in its first shell, 8 in its second, 8 in.
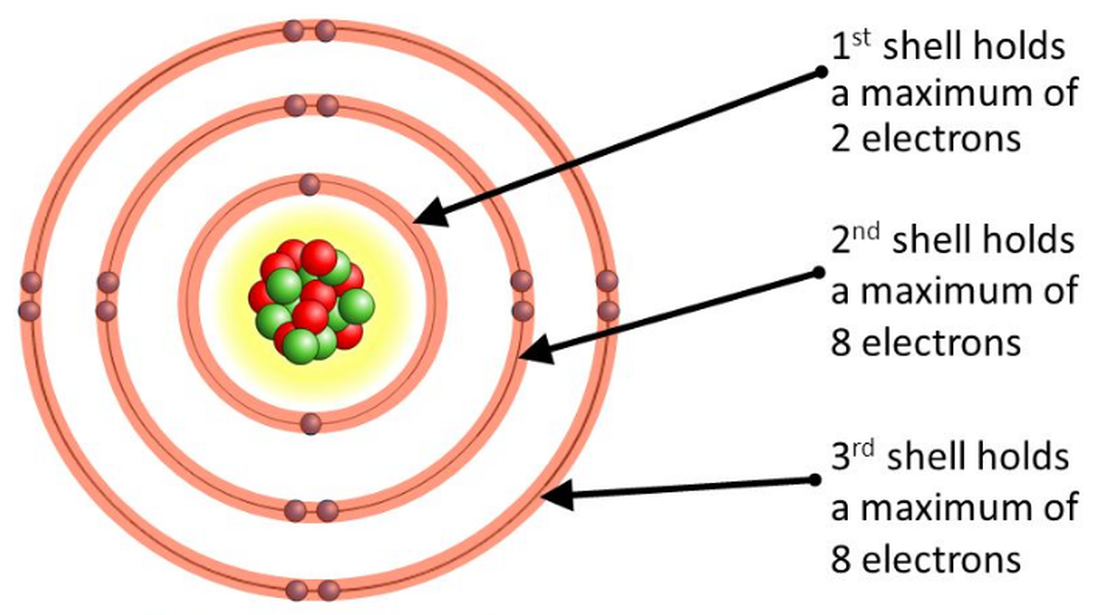
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
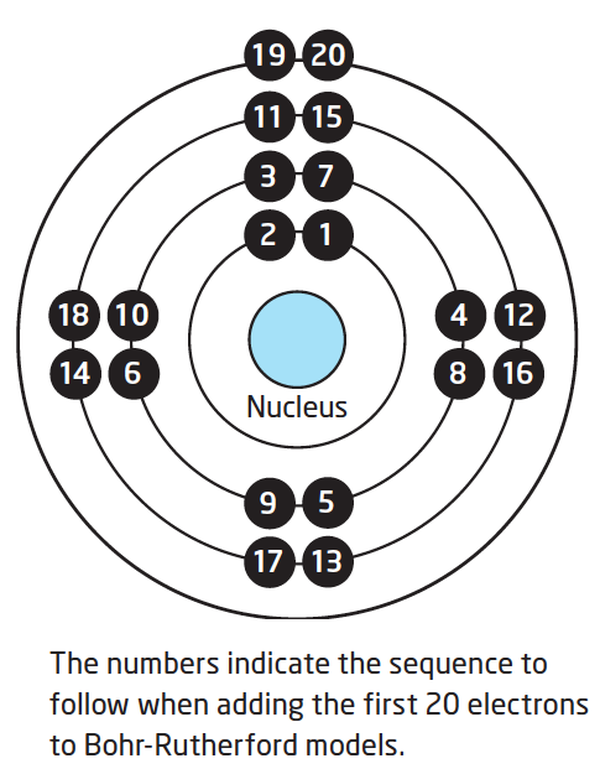
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Bohr Rutherford Diagram For The First 20 Elements
How to draw a Bohr-Rutherford Diagram? Draw a nucleus -write the number of protons and neutrons inside the nucleus. Draw orbitals around the nucleus. Represent electrons as pairs of dots in the orbitals. Draw electrons as dots on the rings that represent the energy levels. Each ring has a maximum number of electrons that it can hold.

bohr rutherford diagram
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.

BohrRutherford Diagrams YouTube
0:00 / 5:40 Bohr-Rutherford Diagram of NaCl (sodium chloride, table salt) chemistNATE 260K subscribers Subscribe Subscribed Share 6.7K views 3 years ago NaCl, sodium chloride, is an IONIC.

PPT Bohr Rutherford Atomic Model PowerPoint Presentation, free
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
PPT BohrRutherford Diagrams for Atoms PowerPoint Presentation, free
Because the Bohr Model is a modification of the earlier Rutherford Model, some people call Bohr's Model the Rutherford-Bohr Model. The modern model of the atom is based on quantum mechanics. The Bohr Model contains some errors, but it is important because it describes most of the accepted features of atomic theory without all of the high-level.

Bohr Rutherford Diagram For First 20 Elements Diagram For You
Silicon has 2 electrons in its first shell, 8 in its second, 4 in its third.Check me out: http://www.chemistnate.com

Bohr Atomic Model Of Hydrogen
This Bohr-Rutherford model explains the structure of the atom, placement of different atomic species inside the atom as well as the charge on different atomic particles. It also explained why electrons remain confined to their shells instead of falling inside the nucleus.
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
Non-Metals. Non-metals - Tend to have 4, 5, 6, or 7 electrons in their outer orbits (shells). They gain electrons to form negative ions (anions) They gain electrons, thus they have the same electron arrangement as the Noble gas in the same row. Try to make a Bohr-Rutherford ion for phosphorous. 15 31 P.
Bohr Model Atom Electron Shell Copper Rutherford Elements Vector
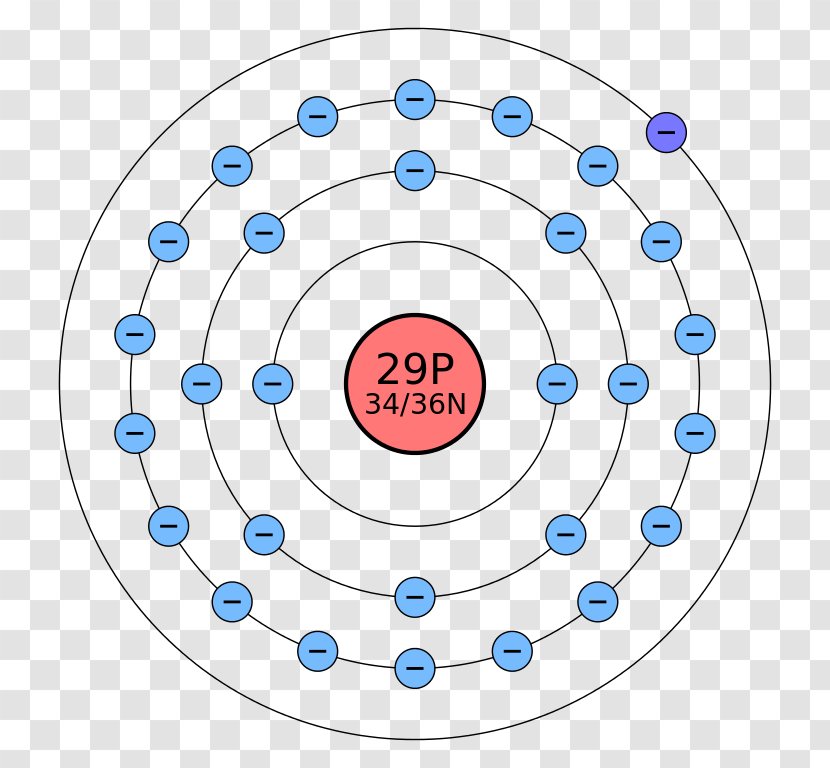
A Bohr Rutherford diagram, also known as a Bohr model or Rutherford model, is a visual representation of an atom's electron configuration. It shows the arrangement of electrons in the various energy levels or shells surrounding the nucleus of an atom. To create a Bohr Rutherford diagram, follow these steps: